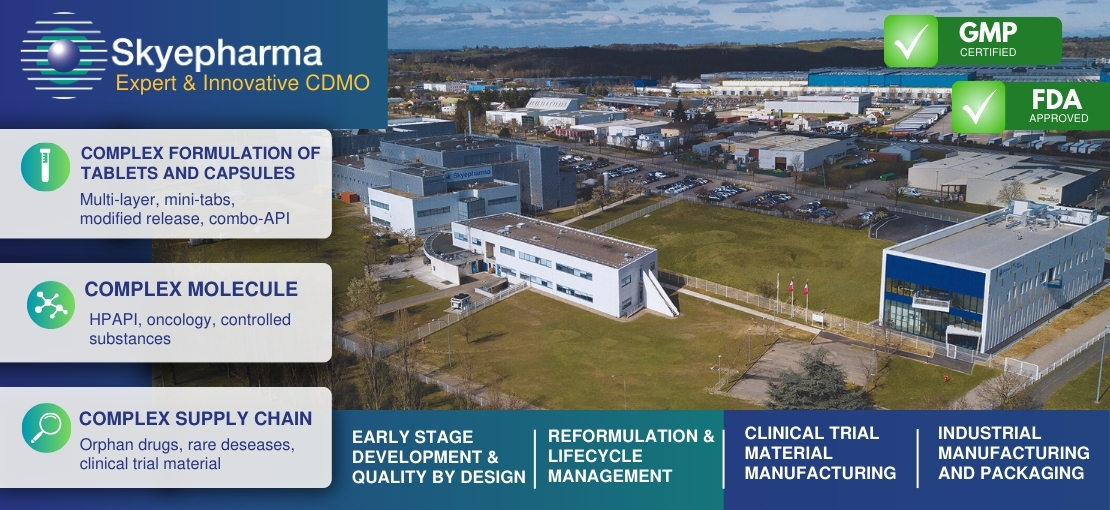
Skyepharma : expert & innovative CDMO | New HPAPI area opening
Skyepharma, a french pharmaceutical service provider (CDMO) with over 25 years of expertise in complex solid forms. Its range of services covers all kinds of needs for pharmaceutical product development from R&D to manufacturing and packaging for international markets.
This year, Skyepharma is launching a new segregated and contained FDA & GMP area dedicated to HPAPI products, including OEB4/5 molecules, NCEs, and immuno and oncology drugs. This area will provide clients with a wide range of services such as R&D, clinical supply, and small to mid-size commercial production (up to 70 kg batch size).
Skyepharma has a strong reputation in development and manufacturing of solid dosage forms like tablets and capsules. Its agility and expertise, particularly in handling highly potent APIs and controlled release techniques, position Skyepharma as a leader in the field of innovation, benefiting its clients and partners.
Type: New Capacity
Category:
CMC: Biologics, Drug Product
CMC: Biologics, Analytical
CMC: Biologics, Drug Delivery
CMC: Biologics, Packaging
CMC: Small Molecules, Drug Product
CMC: Small Molecules, Analytical
CMC: Small Molecules, Drug Delivery
CMC: Small Molecules, Packaging
Hashtags: #api, #bioproduction, #cdmo, #cdmohpapi, #cgmp, #development, #drugdelivery, #drugmanufacturing, #drugproduct, #drugproductcdmo, #drugsubstance, #highpotency, #hpapi, #nonsexualhormones, #orphandrugs, #skyepharma, #smallmolecule
